September 01, 1987 Filed in:
Demo CornerJim Hunt, Guelph Physics Department
This marks the first appearance of this column, which has been prompted by the great popularity of the demonstration sessions at our annual conference. This first column is adapted from an article in the Guelph Daily Mercury
, by Jim Hunt of the Guelph Physics Department.
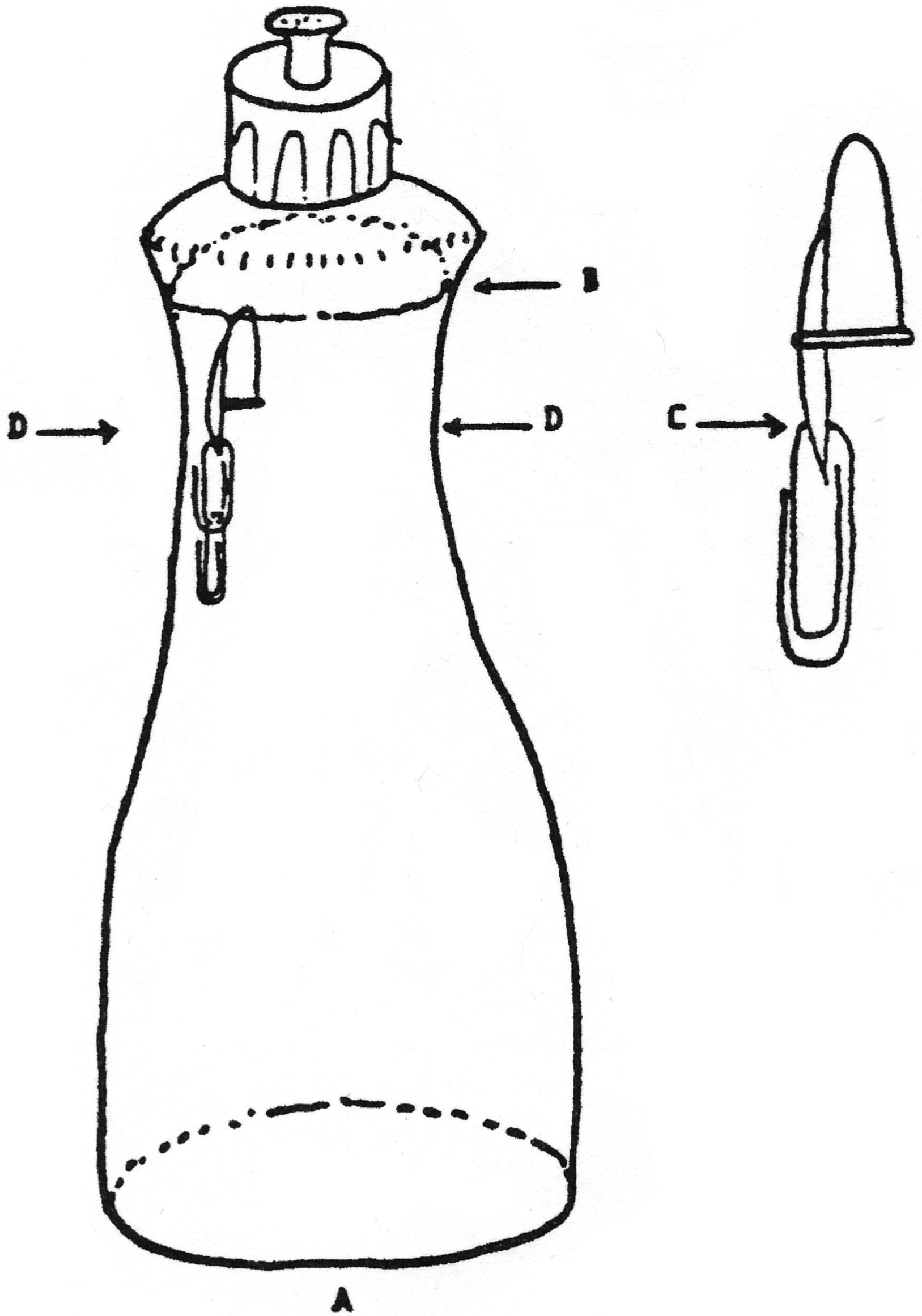
A fun toy which teaches a lot about hydrostatics and Archimedes’ principle can be made from some very simple items. You will need 1) a large transparent dishwashing detergent plastic bottle (see A in Fig.) with a plastic valve cap, and most importantly, with an oval cross section; 2) a cap from a ball point pen; 3) a few small paper clips.
First thoroughly clean the detergent from the bottle and valve and fill the bottle with water to within 2-3 cm of the top (B in Fig.).
Drill a small hole in the pocket clip of the pen cap and hang a paper clip in the hole (C in Fig.). Put this “diver” in a pot of water; with one clip the diver will float easily. The job is now to hang additional paper clips on the first one until the diver just barely does not sink. You might have to trim a bit off the last clip with wire cutters. The model tested for this project required in total two 3-cm clips and three 2-cm clips.
The “just floating” diver should be carefully floated in the water in the bottle, the cap replaced, and the valve closed.
If you now squeeze the bottle gently in its narrowest direction, the trapped air in the diver will be compressed and the diver will sink. Release the pressure and the diver will rise. You have now constructed a standard “Cartesian Diver”, which is interesting enough in itself.
However, we want to go further. Open the valve and put your mouth over the cap and blow — the diver sinks. Now comes the tricky part — with your mouth over the cap and the diver sunk by blowing, close the valve either with your tongue or with a finger also inserted in your mouth and sealed with your lips (we said it was tricky!) The diver now stays on the bottom Instead of the top.
Here comes the surprise — gently squeeze the bottle at the wide part of the “waist” (D in Fig.) and the diver will rise! If it does not rise, you blew too hard originally and will have to try again. With a bit of fiddling around with your initial blowing pressure, you should be able to arrive at a situation in which the diver will rise from the bottom with a squeeze at the wide part of the “waist”, and will sink from the top with a squeeze in the narrow direction.
The valve always leaks somewhat and the pressure will have to be adjusted periodically. You can make a more permanent arrangement with a stopcock replacing the original valve.
How it works
When the diver is floating at the top, squeezing the bottle in the narrow direction compresses the air trapped in the diver, the buoyancy is reduced and the diver sinks. At the bottom of the bottle the hydrostatic pressure of the water is enough to keep the air in the diver compressed so it cannot rise. But the surprising thing about these particular bottles is that squeezing them in their wide dimensions does not decrease their internal volume, but increases it. Thus, with the diver at the bottom, a squeeze at D decreases the pressure in the bottle and causes the air inside the diver to expand with the result that the diver floats to the top.
Another demonstration which involves bottle-squeezing requires a small glass liquor bottle (a “mickey”), topped by a tight-fitting holed stopper with a fine capillary tube inserted in the hole. Squeezing the bottle in its narrow direction causes liquid to flow up the tube; squeezing in the wide direction causes liquid to flow down the tube. Students are often surprised to learn that a solid such as glass is deformable.

Column Editor: Ernie McFarland, Physics Department, University of Guelph, Guelph, Ontario, N1G 2W1 Tags: Pressure


