Dr. Eknath V. Marathé, St. Catharines, Ontario
Spectroscopy
1,2,3 has contributed to our knowledge not only of Earth but also of the Sun, interstellar space, distant stars and galaxies. The subject of spectroscopy began in the year 1666 with the discovery by Newton that when the Sun’s rays are allowed to pass through a prism, they produced a band of colours which he called a spectrum. In 1802, William Hyde Wollaston, (1766 – 1828, English chemist and physicist) used a narrow slit as a secondary source of light and observed dark lines in the spectrum of sunlight. Wollaston thought that the dark lines were natural boundaries between various colours of the spectrum.
In 1814, Josef von Fraunhofer (1787 – 1826, German physicist and optician) also used a slit as a secondary source of solar light. He measured the position of the prominent dark lines and indicated them by the letters A to K (Fig. 1). He determined the wavelengths of the lines, and found that these lines always fell in the same part of the spectrum, whether it was the direct light of the Sun or reflected sunlight from the Moon or planets.
More than half a century passed before a satisfactory explanation of the Fraunhofer lines was given. Léon Foucault (1819 – 1868, French physicist) showed that when light from a very powerful arc was allowed to pass through a sodium flame or vapour located just in front of the slit of a spectroscope, two black lines appeared in exactly the same position of the spectrum as the two D lines of the Sun’s spectrum. If the arc light is removed, the D lines (two yellow lines) appear at the same position of the previous dark lines. This is known as the Reversal of the Spectral Lines. The emission of light by the sodium vapour is rather weak, and when the bright white light is shining through the sodium vapour, the sodium emission spectrum is overpowered by the strong absorption of light, and the dark lines show up in contrast to the bright continuous spectrum in the adjacent yellow region of the spectrum.

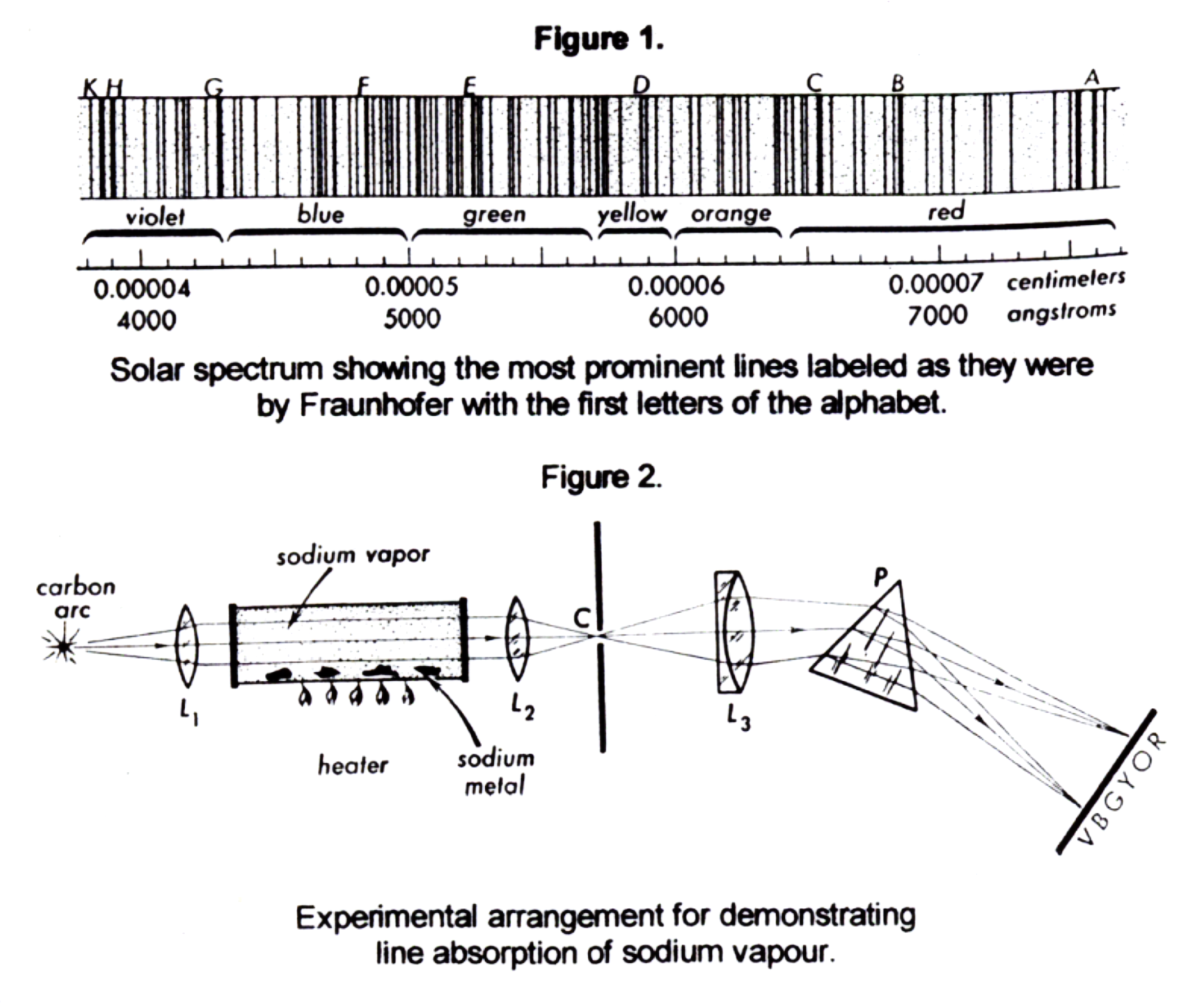
Figure 2 shows the classic apparatus to demonstrate absorption by sodium vapour. L1 and L2 are condensing lenses to produce a parallel beam of light and to illuminate the slit C. Sodium vapour is produced by inserting a small amount of metallic sodium in a partially evacuated tube and heating it with a small gas burner3. As the metal vapourizes and fills the tube with sodium vapour, a dark line appears in the yellow region of the spectrum.
Best results are obtained nowadays by using a bright white light that can be focused into a beam (such as used in a slide projector) and a sodium flame generated by placing rock salt on a Meeker burner. A spectrometer is the best device for observing the spectrum.
Endnotes
1 White, Harvey Elliot,
Introduction to Atomic Spectra, McGraw-Hill Book Company, Inc., New York, 1934, pp. 1 & 2.
2 White, Harvey E.,
Physics – An Exact Science, D. Van Nostrand Company, Inc., New York, 1959, Library of Congress
Card No. 59-12778, pp. 459-463.
3
Physics Demonstration Experiments, Edited by Harry F. Meiners, Vol. 2, Ronald Press Co. for American Association of
Physics Teachers, 1970, pp. 1203-1204.
________________________________________________________________________________________________
Column Editor: Ernie McFarland, Physics Department, University of Guelph, Guelph, Ontario, N1G 2W1 Email: elm@physics.uoguelph.ca
Submissions describing demonstrations will be gladly received by the column editor

Column Editor: Ernie McFarland, Physics Department, University of Guelph, Guelph, Ontario, N1G 2W1 Tags: Light


