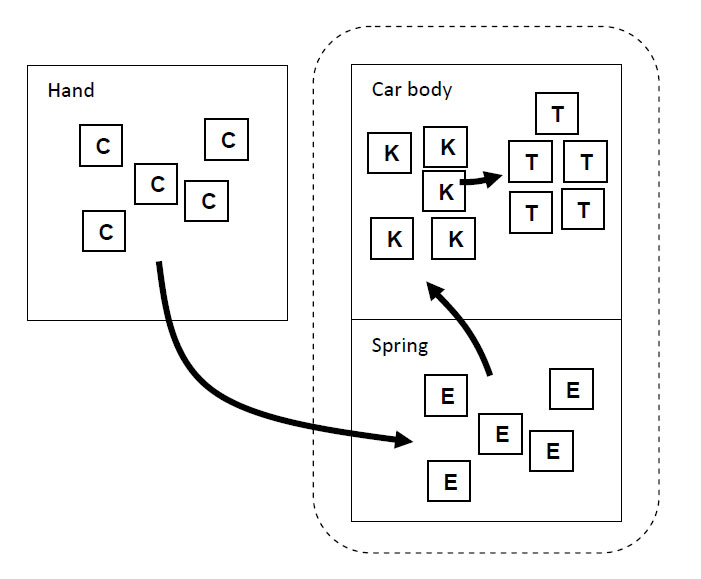
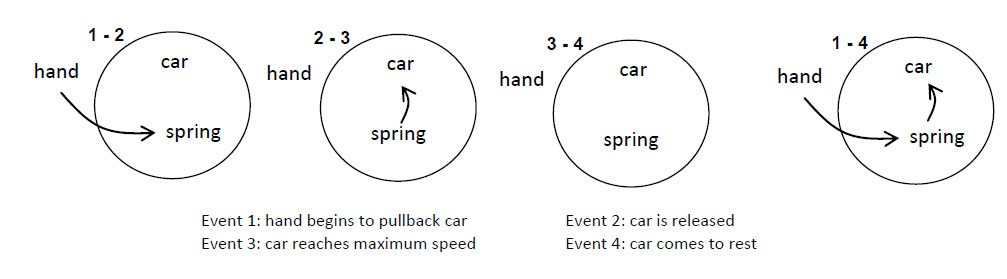
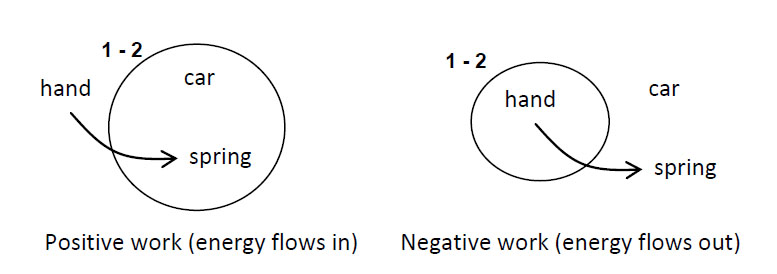
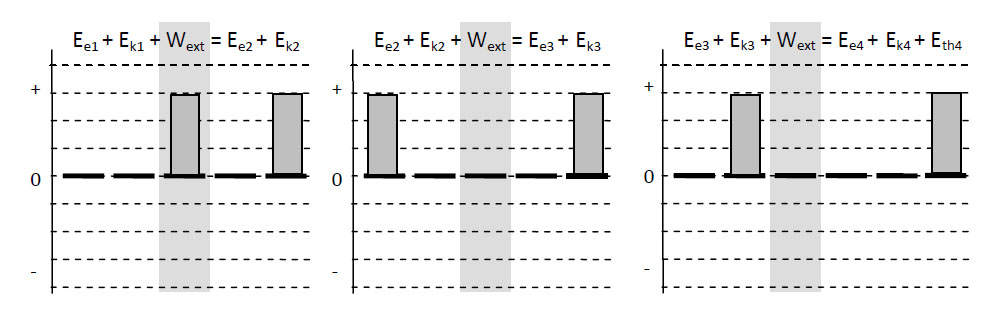Imagine a child, perhaps “Dennis the Menace,” who has blocks which are absolutely indestructible, and cannot be divided into pieces. Each is the same as the other. Let us suppose that he has 28 blocks. His mother puts him with his 28 blocks into a room at the beginning of the day. At the end of the day, being curious, she counts the blocks very carefully, and discovers a phenomenal law—no matter what he does with the blocks, there are always 28 remaining! This continues for a number of days, until one day there are only 27 blocks, but a little investigating shows that there is one under the rug—she must look everywhere to be sure that the number of blocks has not changed. One day, however, the number appears to change—there are only 26 blocks. Careful investigation indicates that the window was open, and upon looking outside, the other two blocks are found. Another day, careful count indicates that there are 30 blocks! This causes considerable consternation, until it is realized that Bruce came to visit, bringing his blocks with him, and he left a few at Dennis’ house. After she has disposed of the extra blocks, she closes the window, does not let Bruce in, and then everything is going along all right, until one time she counts and finds only 25 blocks. However, there is a box in the room, a toy box, and the mother goes to open the toy box, but the boy says “No, do not open my toy box,” and screams. Mother is not allowed to open the toy box. Being extremely curious, and somewhat ingenious, she invents a scheme! She knows that a block weighs three ounces, so she weighs the box at a time when she sees 28 blocks, and it weighs 16 ounces. The next time she wishes to check, she weighs the box again, subtracts sixteen ounces and divides by three. She discovers the following:
number of blocks seen + (weight of box − 16 ounces)/3 ounces = constant
There then appear to be some new deviations, but careful study indicates that the dirty water in the bathtub is changing its level. The child is throwing blocks into the water, and she cannot see them because it is so dirty, but she can find out how many blocks are in the water by adding another term to her formula. Since the original height of the water was 6 inches and each block raises the water a quarter of an inch, this new formula would be:
number of blocks seen + (weight of box − 16 ounces)/3 ounces + (height of water - 6 inches)/¼ inch = constant.
In the gradual increase in the complexity of her world, she finds a whole series of terms representing ways of calculating how many blocks are in places where she is not allowed to look. As a result, she finds a complex formula, a quantity which has to be computed, which always stays the same in her situation.2