Michelle Lee, Lisgar C. I.
michelle.lee@ocdsb.ca
Roberta Tevlin,Retired
roberta@tevlin.ca
Understanding the greenhouse effect is critical to understanding climate change and PhET has two excellent simulations that can help. This article describes how you can use these two simulations and a couple of supporting videos, to help your students develop a good understanding of the topic. This is most obviously relevant to the grade 10 Climate Change Unit. It is also relevant to grade 9 Astronomy and Ecosystems, Grade 10 Optics, and senior chemistry and physics.
Before looking at the simulations, students should see a real example of greenhouse gases. This short video from the Perimeter Institute provides a very simple, clear demonstration.
This video shows four different gases in transparent containers that are held over a heating pad. An infrared camera is used to show that CH
4 and CO
2 absorb the heat from a heating pad, like the towel in the video did, while O
2 and N
2 do not.
Infrared light is not special. Different wavelengths are absorbed differently by different materials. This next video provides a couple of good demonstrations of this.
It shows that glass is transparent to visible, but not infrared, and that a black garbage bag is transparent to infrared, but not visible light. This video reinforces that if light (visible or infrared) is absorbed, then it will look darker to your eyes or a camera.
To explain the observations in these videos, we begin with the first of two PhET simulations. This PhET simulation will help students build a mental model of what happens when light is absorbed and re-emitted by molecules.
PhET Molecules and Light
This simulation starts with infrared light and carbon monoxide. Push the green button to start the flow of infrared photons. Sometimes nothing happens. Sometimes the light is absorbed. When that happens, the molecule vibrates for a while and then infrared light is emitted in a random direction.
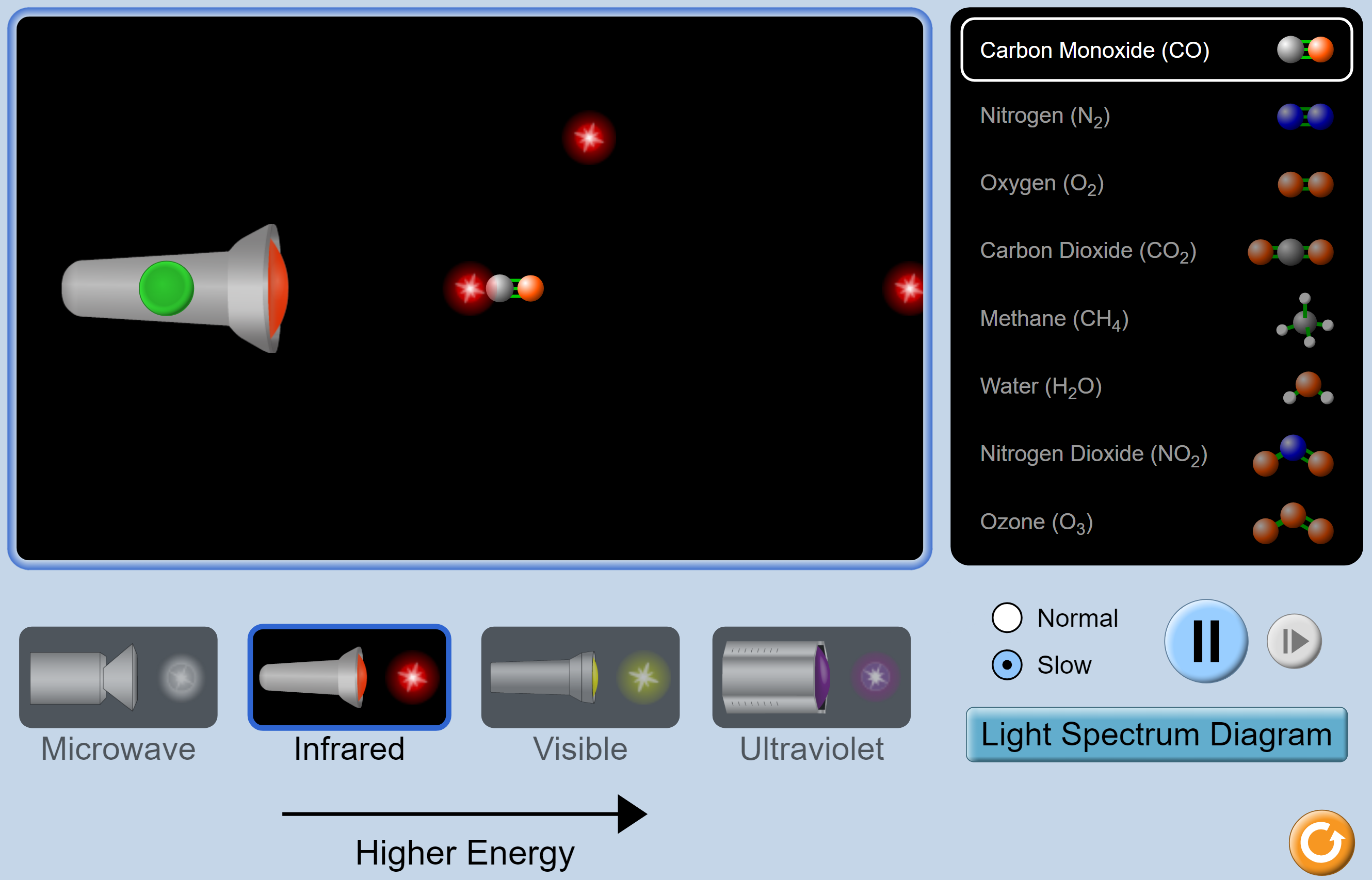
There are seven other gases that can be tested: N
2, O
2, CO
2, CH
4, H
2O, NO
2, and O
3. Ask the students to predict what will happen in each case. From the video, they should be able to predict that nothing will happen with the O
2 and N
2, and that the CO
2 and CH
4 will act like the CO. Using the simulation, they will see that they were right. They don’t have the background information to predict what will happen to H
2O and O
3, but the simulation will show them that these molecules also absorb and re-emit the light.
Next, you should use the simulation to test the different gases with visible light. Have the students make predictions first. These predictions might need more time and perhaps some extra probing questions, such as:
- Are the air molecules around you right now absorbing and re-emitting the light in a random direction, or are they letting the visible light through? Students should recognize that we can see clearly through the air, which means that the gases in the air do not absorb visible light.
- So, what molecules are in the air? They should know that oxygen, carbon dioxide and water are there, and they may also remember about nitrogen. As predicted, there are no interactions between these four gases and visible light.
- What about the other three (CH4, NO2, and O3)? These other gases all happen to be bad for your health and they show up when the air is polluted or ventilation is poor. However, they are also transparent to visible light, with one exception. In the simulation, nitrogen dioxide absorbs the light, and this makes the molecule glow — not vibrate. It is not clear what the PhET demo is trying to show with this. Nitrogen dioxide is the gas that makes summer smog look brownish in colour and it can form secondary particles called nitrates that cause haze and reduce visibility. So this might be what the glowing is supposed to indicate.
If you have time, students could explore the effect of microwave radiation on the molecules. It is absorbed by some molecules, but it causes them to rotate instead of vibrate. You will also see that ultraviolet light causes the O
3 and NO
2 molecules to lose an oxygen atom and turn into O
2, N
2 and some very reactive oxygen atoms. This is how the ozone layer protects us from UV light. This extra information will probably distract from the understanding of the greenhouse effect but might be useful in another course.
At this point, the students will have realized that visible light passes through most of the gasses unaffected and that five of the seven absorb and re-emit infrared light. The gases that absorb and re-emit infrared radiation are called greenhouse gases. We will see why they are called this and what this means for the Earth with the next PhET Greenhouse Effect simulation.
PhET Greenhouse Effect 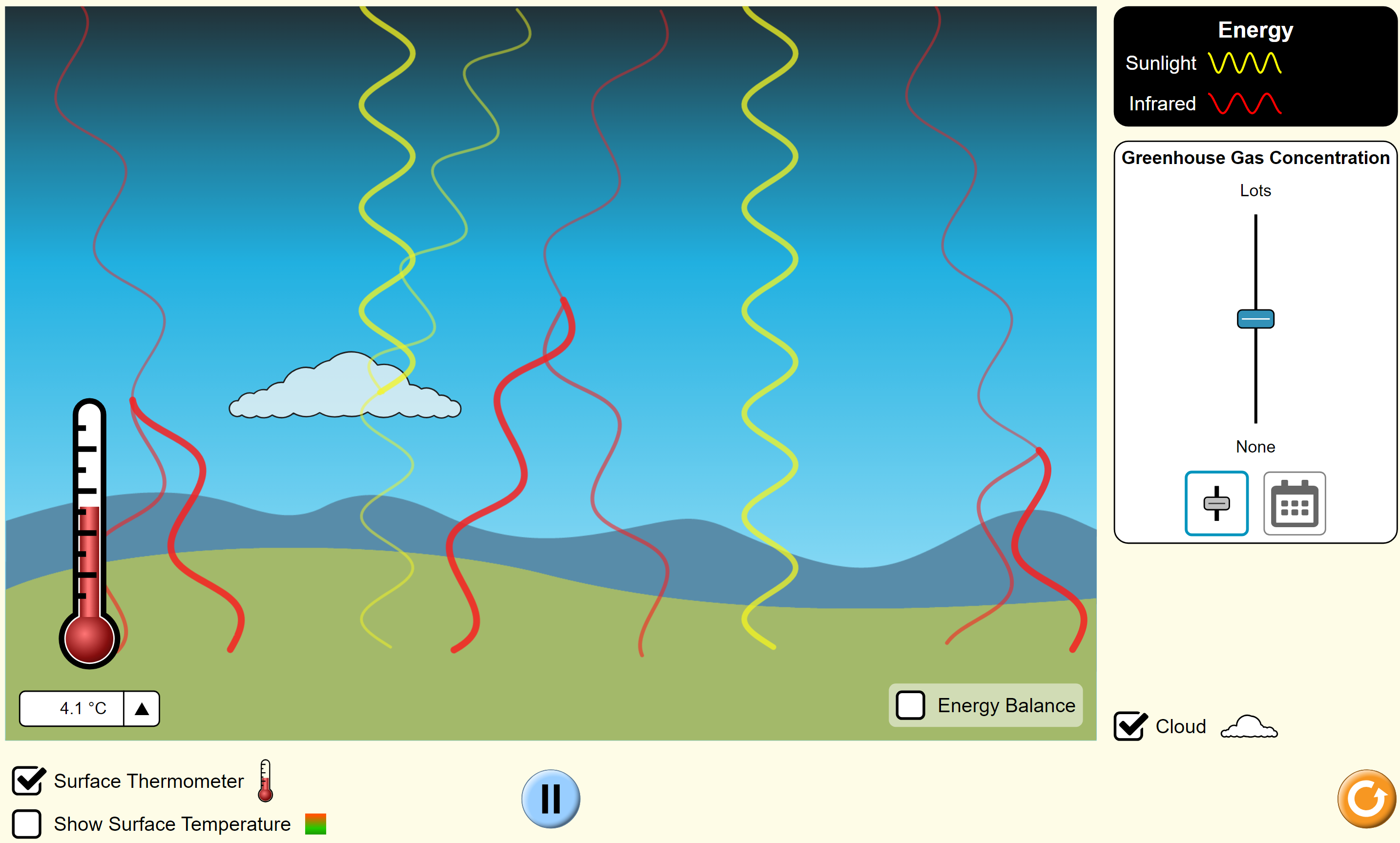 General Observations
General Observations
When you open
this simulation choose ‘waves’, select ‘cloud’, and click on ‘start sunlight’. Do not change the setting on Greenhouse Gas Concentration. Have the students work in small groups, making point-form notes on a whiteboard (or equivalent) of all the different things they notice or wonder. Encourage them to come up with at least six ideas. Here are some possibilities:
- Yellow light is coming down to the Earth, probably from the Sun.
- Some yellow light reflects off the cloud and some passes through.
- Red (infrared) light is going out from the Earth.
- Some of the infrared light is sent back to the Earth.
- Yellow light does not reflect off the Earth.
- Maybe the yellow light hitting the Earth is absorbed.
- The infrared light does not appear until the yellow light hits the Earth and disappears.
- Maybe the infrared light comes from the absorbed yellow light.
- The infrared light has a longer wavelength than the yellow light.
- There are more infrared waves than yellow waves. Maybe yellow light has more energy?
- The temperature started at –28.1 C (they will probably miss this; you can show this by restarting with the refresh button).
- The temperature rises quickly and then slower and slower.
- After some time, the temperature eventually stays constant at 4.4 C.
After they have had enough time to collect observations, you need to consolidate the ideas. One way is to have one person from each group give an observation. The other groups write this down or they put a check mark if they already have it. One by one, each group gets a chance to give an observation but only if it is a new one. This consolidation will give a chance for students to appreciate the complexities and limitations of this simulation.
Connecting to Infrared light absorption and re-emission
Have the students use their new knowledge to answer the following concept question.
What is causing the infrared light to return to the Earth?
- Gravity is pulling it down.
- It is reflecting off water droplets in clouds.
- It is reflecting off molecules of air.
- It is being absorbed and re-emitted by molecules in the air.
This is not a simple factual-recall multiple-choice question. Give them time to think and then discuss their answers in small groups.
Assuming they have not looked at general relativity, they should be able to eliminate answer A.
Answer B is reasonable and in reality, infrared light does reflect from the underside of clouds. However, the simulation only shows visible light reflecting from the top of the clouds and so the simulation will cause them to eliminate this answer.
They now just have to decide if this looks like answer C (reflection) or answer D (absorption and re-emission). Unfortunately, the simulation will once again mislead them. The simulation makes it look like the infrared light is reflecting off something, because it always heads back to Earth. To show that it is caused by absorption and emission, the light should be emitted in random directions, where only some of the light heads back to the Earth. This effect was shown in the first simulation. However, for this simulation the designers tried to make the image clearer, but in doing so they made it less accurate.
In the real world, the correct answer would be B and D, and D is the greenhouse effect. Have your students draw a diagram that includes the random emissions. I think having the students critique this overly-simplified simulation will help them understand the reality better.
If you have time, and are philosophically inclined, you could have a wide-ranging discussion of how all simulations and models have limitations and are simpler than the phenomena. You could also read to them what Jorge Luis Borges and Lewis Carroll have to say about the topic here,
On Exactitude in Science.
Exploring the Energy Balance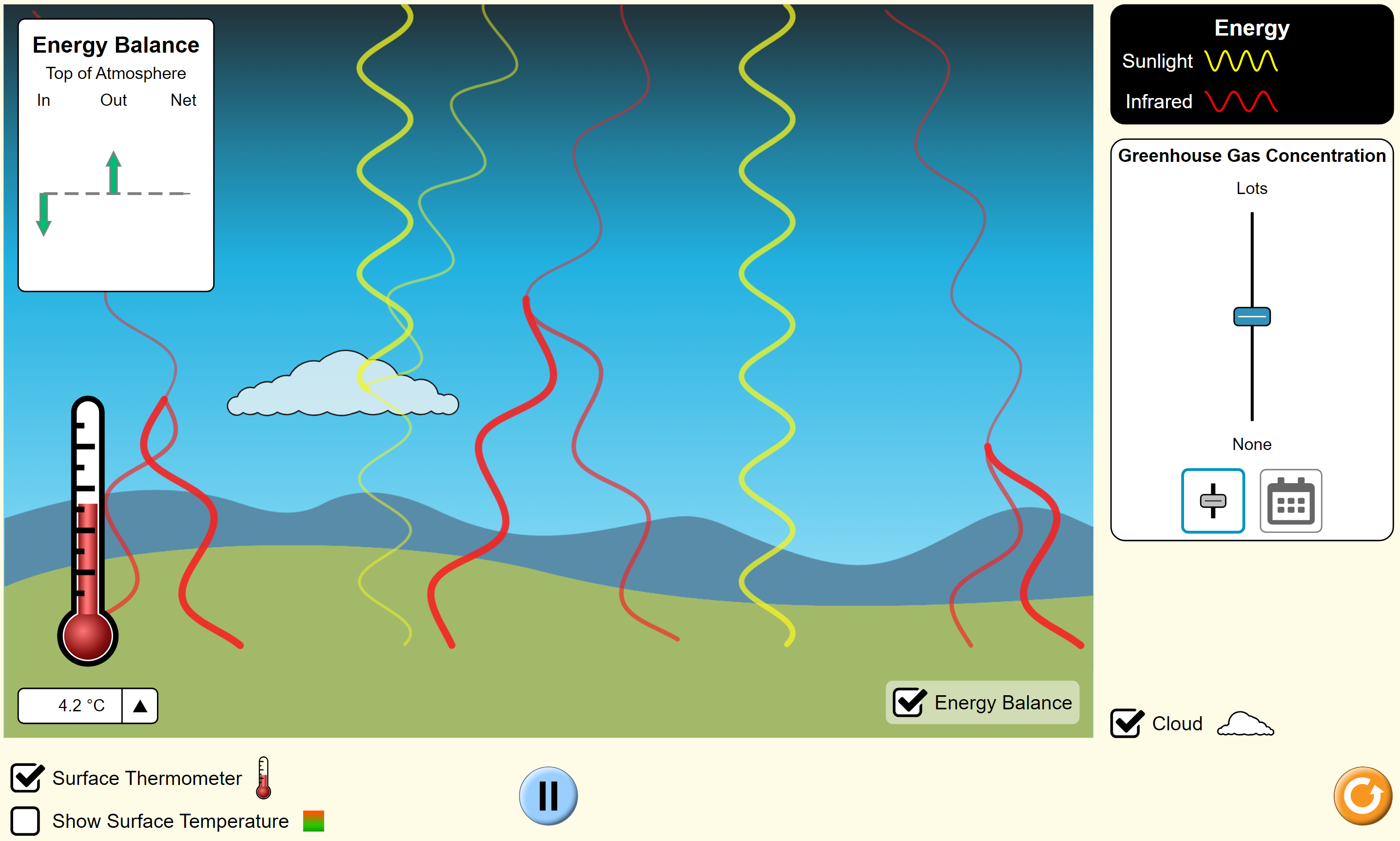
Next, click on ‘energy balance’ and students will see that the same amount of energy is coming in as is going out. Note that the system is currently at equilibrium as it has been running for a while.
Next, refresh the simulation by clicking the orange button at the bottom right. Select ‘energy balance’ and then ‘start sunlight’. What do you notice about the energy balance and temperature? Again, have the students make point-form notes. They should see the following:
- At the start there is no energy going out, it is just coming in.
- Later more and more energy goes out until it is equal to the energy coming in.
- The net energy goes to zero when the energies in and out are equal.
- The net arrow goes almost to zero quickly, and then shrinks at a slower and slower rate. It is hard to see when it becomes exactly zero.
- The temperature rises when there is more energy coming in than going out.
- The temperature stops rising when the net energy becomes zero.
- The extra energy that came in at the start is stored as a higher temperature.
Notice that greenhouse gases don’t trap the infrared light permanently, as many explanations seem to suggest. Instead, they just cause the energy to stay around in the atmosphere longer and this results in a higher temperature.
Changing the Amount of Greenhouse Gas Concentration
Next, refresh the simulation. Click on ‘energy balance’. Move the slider for greenhouse gasses to the highest value. Predict what will be different. Run the simulation. What do you notice?
- The final temperature is higher, 25.7 C.
- The net energy took longer to get to zero.
Historical Times
Finally, choose the ‘Calendar Icon’
📅 instead of the greenhouse gas slider. This allows you to see the situation at four different times in Earth’s history. Predict how the four situations will be different. Try guessing what the final temperatures will be.
- The ice age will be coldest, probably because of less greenhouse gases (7.5 C).
- 1750 is just before the industrial revolution and will be the next coldest (13.6 C).
- 1950 will be warmer (13.8 C, only slightly warmer).
- 2020 will be even warmer (14.9 C).
Extensions for Senior Physics Courses
At this point, grade 10 students should have a pretty clear idea of how gases in the atmosphere can cause a rise in temperature through the greenhouse effect. What has not been explained is why certain molecules absorb infrared and others do not. An explanation of this is more suited for a grade 12 physics class. However, this video does a quick job of explaining it (watch 0:29 - 02:19).
For a more in-depth explanation and how you can connect this in a physics course you should download the free resource
Beyond Bohr from the Perimeter Institute.
Resources for Teaching Climate Change
We are developing resources to help you and your colleagues teach climate change in grade 10 science and in senior physics courses. In order to make these lessons really useful, we need other teachers to try them out, either the whole thing or just parts of a resource. We need teachers who will let us know what worked and what didn’t and how they modified the lessons. If you are interested in being part of this effort or would just like early access to these lessons, please fill out
this Google form and we will get back to you.
Thanks in advance from James Ball, Iain Braithwaite, Michelle Lee, Robert Prior, Milica Rakic, Dale Simnett, and Roberta Tevlin.
Tags: Climate, Energy, Light, Waves and Sound




